INLTV Uncensored News
INLTV is Easy To Find Hard To Leave
Click Here for the best range of Amazon Computers
Click Here for INL News Amazon Best Seller Books
Amazon Electronics - Portable Projectors
Whuhan Lab China - Special Sky News Covid-19 Investigation
Bombshell dossier indicates China has been
‘preparing’ for bio-warfare" ... Allan Jones
A Word on the SARS-CoV-2 Vaccine a
How is it that a vaccine for the SARS-CoV-2 virus, which under normal conditions would take years to develop, was promptly launched in early November 2020? The vaccine announced by Pfizer is based on an experimental gene editing mRNA technology which has a bearing on the human genome.
Were the standard animal lab tests using mice or ferrets conducted?
Or did Pfizer “go straight to human “guinea pigs.”? Human tests began in late July and early August. “Three months is unheard of for testing a new vaccine. Several years is the norm.”
This caricature by Large + JIPÉM explains our predicament:

Mouse No 1: “Are You Going to get Vaccinated”,
Mouse No. 2: Are You Crazy, They Haven’t finished the Tests on Humans”
Barely reported by the media: “Six people died in Pfizer’s late-stage trial of the COVID-19 vaccine, the FDA revealed just hours after Britain became the first country in the world to roll out the vaccine.”
“Rest assured”, the vaccine is “safe”. According to the FDA:
the deaths are said to raise no new safety issues or questions about the vaccine’s effectiveness”.
French drug assessment center demands removal of all four widely used COVID vaccines
April 22, 2021 (LifeSiteNews) — A regional independent drug assessment center, the CTIAP (Centre territorial d’Information indépendante et d’Avis pharmaceutiques), which is linked to the Cholet public hospital in the west of France, recently published a report showing that the vaccines used against COVID were not only submitted to insufficient clinical testing, but that the quality of the active substances, their “excipients, some of which are new,” and the manufacturing processes are problematic. “These new excipients should be considered as new active substances,” the Cholet hospital team stated, in a study that according to them raises issues that have not been commented to date.
The team led by Dr. Catherine Frade, a pharmacist, worked on public data released by the EMA with relation to the Pfizer, Moderna, AstraZeneca and Janssen (Johnson & Johnson) shots, and its first caveat was that all these products only have temporary marketing authorizations. They are all subject to further studies that reach as far as 2024 and even beyond, and these will be almost impossible to be completed because of the way the vaccines are now being distributed, said the CTIAP report.
These studies even include the stability and comparability of the vaccine batches put on the market and the quality and safety of excipients — substances formulated alongside the active ingredient of a medication to facilitate or enhance their absorption.
According to the CTIAP, all of the vaccines were put on the market and actively used on human beings before “proof of quality for the active substance and the finished product” was produced: all the manufacturing labs obtained future deadlines to submit their studies in this regard.
The authors of the report consider that the “variabilities, which impact the very core of the product, could even invalidate any clinical trials conducted” in the coming months and years.
They go so far as to state: “Prudence would even dictate that, in all countries where these vaccines against COVID-19 have been marketed, all the batches thus ‘released’ should be withdrawn immediately; and that these MAs that have been granted should be suspended, or even canceled, as a matter of urgency until further notice.”
Here below is LifeSite’s full working translation of the CTIAP’s April 2 report:
Can we imagine launching a car manufacturing line and putting vehicles on the road, despite the uncertainties noted in the official documents published? These uncertainties are related to the quality of the parts making up the engine and the various other parts, including those related to safety, the manufacturing process, the reproducibility of the batches that are being marketed, etc.
In the field of medicines (including vaccines), the pharmaceutical act of “release” of the finished product (an authorized product intended for sale) constitutes the final stage of control that precedes the release of these products to the population. This key step of “release” is under the pharmaceutical responsibility of the manufacturers.
Following its previous analyses, the CTIAP of the Cholet Hospital Center has once again revealed to the public, and probably in an unprecedented and exclusive way, new vital information concerning the following four vaccines against COVID-19: the one from the BioNTech/Pfizer laboratory; the one from the Moderna laboratory; the one from the Astra Zeneca laboratory; the one from the Janssen laboratory.
This work was made possible thanks to the valuable contribution of Dr. Catherine Frade, pharmacist and former director of international regulatory affairs in the pharmaceutical industry. She graciously provided us with a documented, written alert. In this document, she sheds light on data extracted, on March 22, 2021, from the MA (marketing authorization) itself; an MA qualified as “conditional.” She has extracted “source data that is difficult to identify by someone who does not work in the field.” This data is therefore public and verifiable. First of all, it should be noted that the author of this document no longer works in the pharmaceutical industry; she states: “First of all, I would like to make it clear that I have no conflict of interest with the pharmaceutical industry.” It is therefore with her agreement that CTIAP intends to make available to the public, health professionals, decision-makers … an analysis of some of these data that all should read carefully.
This reflection first presents what a “conditional” MA is (I). Then, it recalls that the studies for these vaccines are not complete, as they run from “2021 to at least 2024” (II). Then, it reveals, in an unprecedented and exclusive way, that the official documents, published by the European Medicines Agency (EMA), underline the insufficiency of the evidence concerning also the “quality” of the “active substance” and of the “excipients,” of the “manufacturing process,” of the “reproducibility of the batches” that are being commercialized, etc. (III). Finally, this analysis proposes a conclusion.
I — First of all, it is important to understand what a “conditional” MA is
An MA is to a drug what a car registration document is to a car. MA is granted when a drug has proven its quality, efficacy, and safety; with a positive benefit/risk ratio: that is, it presents more benefits than risks. Obtaining this MA is the essential condition for a pharmaceutical laboratory to sell any drug, including vaccines.
Here, in the case of these vaccines against COVID-19, the four MAs issued are so-called “conditional” MAs. They are temporary. They are valid for no more than one year, because they were obtained on the basis of “incomplete data.” To obtain a standard 5-year MA, the laboratories concerned must provide dossiers completed with “studies in progress and studies planned for the coming years.” Throughout “this development,” close and coordinated monitoring between the manufacturing laboratories and the health authorities is organized through regular discussions. The “conditional” MA is “re-evaluated each year” according to the contribution and critical analysis of additional data provided and collected during a full year.
This “conditional” MA is a European MA. It was obtained through the centralized accelerated procedure. It allows simultaneous marketing in the following 30 countries (European Union and European Free Trade Association): Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden.
The studies concerning these four vaccines are therefore still in progress.
II — Secondly, the planned studies are still in progress and are spread over a period ranging from “2021 to at least 2024”
All of the studies submitted during the MA application are summarized in the EPAR (European Public Assessment Report). This report is published on the European Medicines Agency (EMA) website. The planned studies, not yet completed, are also included.
This schedule, which “extends from 2021 to at least 2024,” depending on which COVID-19 vaccine is involved, is defined in the “annexes” of the conditional marketing authorization and in the published EPARs.
As an example, the BioNTech/Pfizer vaccine received this European conditional MA on December 21, 2020. And the deadline for filing “confirmation” of efficacy, safety, and tolerability of this vaccine is “December 2023.”
The Moderna vaccine was granted marketing authorization on January 6, 2021. The deadline for filing “confirmation” of efficacy, safety, and tolerability of the vaccine is “December 2022” at the earliest.
AstraZeneca’s vaccine was granted marketing authorization on January 29, 2021. The deadline for filing “confirmation” of efficacy, safety, and tolerability of the vaccine is “March 2024.”
The Janssen vaccine was granted conditional European marketing authorization on March 11, 2021. The deadline for submitting “confirmation” of the vaccine’s efficacy, safety and tolerance is “December 2023.”

However, to date — and this is undoubtedly where the unprecedented and exclusive revelation of this study lies — another deadline has been set for these four vaccines. This deadline no longer concerns only the ongoing clinical trials, but also the “proof of quality for the active substance and the finished product” itself: that is, the intrinsic quality (the heart) of the product sold and administered to millions of people.
III — Thirdly, and this seems to be unprecedented, the published official documents also underline the incompleteness of the evidence concerning the “quality” of the “active substance” and “excipients,” the “manufacturing process,” the ”reproducibility of the batches” marketed, etc.
The deadline for submitting additional evidence on the “quality” of the “active substance” and the “finished product” (i.e., the vaccine that is authorized and sold) is set for:
- “July 2021” for BioNTech/Pfizer;
- “June 2021” for Moderna;
- “June 2022” for Astra Zeneca;
- “August 2021” for Janssen.
Indeed, for these 4 vaccines, paragraph E, “Specific obligation regarding post-authorization measures for the conditional marketing authorization,” taken from Annex II of the MA, clearly states the following:
For the BioNTech/Pfizer vaccine (pages 18-19)
By “March 2021,” the laboratory must provide “additional validation data” to “confirm the reproducibility of the finished product manufacturing process.”
By “July 2021,” the laboratory must provide missing information to:
- “complete the characterization of the active substance and the finished product;”
- “strengthen the control strategy, including the specifications of the active substance and the finished product” in order to “ensure the constant quality of the product;”
- “provide additional information regarding its synthesis process and control strategy” in order to “confirm the purity profile of the excipient ALC-0315” and “to ensure quality control and batch-to-batch reproducibility throughout the life cycle of the finished product;”
- and by “December 2023,” and “in order to confirm the efficacy and safety” of this vaccine, the company “shall submit the final clinical study report for the randomized, placebo-controlled, blind observer study (Study C4591001).
For the Moderna vaccine (page 15)
The laboratory should provide the missing information to:
- “complete the characterization of the manufacturing processes of the active substance and the finished product” (deadline “January 2021”);
- confirm the reproducibility of the manufacturing process of the active substance and the finished product (initial and final batch sizes) (deadline “April 2021”);
- “provide additional information on the stability of the active substance and the finished product and review the specifications of the active substance and the finished product after longer industrial practice” with the aim of “ensuring consistent product quality” (deadline “June 2021”);
- “submit the final study report for the randomized, placebo-controlled, blinded clinical trial for the mRNA-1273-P301 observer” to “confirm the efficacy and safety of COVID-19 vaccine Moderna” (by December 2022).
For the Astra Zeneca vaccine (pages 14-15)
The laboratory must submit the missing information in order to:
- “provide additional validation and comparability data, and initiate further testing” with the aim of “confirming the reproducibility of the manufacturing processes of the active substance and the finished product” (by “December 2021”);
- “Provide the main analysis (based on the December 7 data cut-off (post database lock) and the final analysis of the combined pivotal studies” to “confirm the efficacy and safety of COVID-19 Vaccine AstraZeneca” (deadline “March 5, 2021” (for the main analysis) and “May 31, 2022” (for the combined analysis);
- “submit final reports of the randomized controlled clinical studies COV001, COV002, COV003 and COV005” to “confirm the efficacy and safety of COVID-19 Vaccine AstraZeneca” (due "May 31, 2022");
- “provide additional data regarding the stability of the active substance and the finished product and revise the specifications of the finished product after extensive industrial practice” in order to “ensure consistent product quality” (deadline “June 2022”);
- “submit the synthesis and summaries of the primary analysis and the final clinical study report for study D8110C00001” to “confirm the efficacy and safety of COVID-19 vaccine AstraZeneca in the elderly and in subjects with underlying disease” — due “April 30, 2021” (for the primary analysis) and “March 31, 2024” (for the final study report).
For the Janssen vaccine (page 18)
The laboratory should submit the missing information to:
- “provide additional comparability and validation data” to “confirm the reproducibility of the manufacturing process of the finished product” (deadline “August 15, 2021”);
- submit the final report of the VAC31518COV3001 randomized, placebo-controlled, single-blind clinical study to “confirm the efficacy and safety of the COVID-19 Ad26.COV2.S vaccine” by December 31, 2023.
These facts allow us to offer a conclusion.
Conclusion
For these reasons, which are not exhaustive, it has proved useful to look for and read the content of the paragraph E: “Specific obligation relating to post-authorization measures concerning the conditional marketing authorization,” extracted from Annex II of the MA, corresponding to each of these 4 vaccines against COVID-19.
The inadequacy of the evaluation does not only concern the clinical trials (studies conducted in humans (women and men)), but also the quality of the active substance, the excipients, some of which are new, the manufacturing process, and the batches released and administered to humans in several countries around the world.
Moreover, these new excipients must be considered as new active ingredients, and thus be the subject of a complete evaluation file similar to that required for a new active ingredient.
Changing the commercial name of one of these vaccines, as was recently announced for the AstraZeneca vaccine in particular, can only be considered as a cosmetic arrangement of the product’s image for marketing purposes (winning new public confidence, boosting sales). It would not answer the questions raised concerning the quality, efficacy and safety of the product. This is one of the usual techniques used to put make-up on (dissimulate) certain undesirable characteristics of the product concerned. It is a technique that has been used to present other drugs in the best possible light.
As already mentioned, in the field of medicines (including vaccines), the “release” of the finished product (intended for sale) is the final stage of control (of quality and therefore of safety) before making these products available to the population.
This key stage of “release” of batches is the pharmaceutical responsibility of the manufacturers. However, the responsibility of the users (institutions and health professionals in particular) may also be involved.
In our opinion, these clinical studies should never have begun before the intrinsic quality of the finished product and its manufacturing process had been fully mastered; before the formulas of these vaccines had been stabilized.
How can the results of these clinical trials, conducted on a global scale, be compared if the vaccine administered can vary from one manufacture to another, from one batch to another, from one region to another?
These variabilities, which impact the very core of the product, could even invalidate any clinical trials conducted.
Even in the case of a health emergency, it is therefore difficult for us to understand the basis for the MA (marketing authorization) that has been granted to these COVID-19 vaccines.
In addition to the uncertainties related to COVID-19, there are also the approximations related to the use, and the intrinsic quality, of these vaccines. Now two problems will have to be managed instead of one.
The maneuver seems subtle. The useful information is available in the official documents published in the framework of the MA; but this data is not made visible by the official discourse. It seems the latter has only tried to present these products as being effective and safe, without reservations; even though the formulas and manufacturing processes of these vaccines do not even seem to have been fully stabilized yet.
These new revelations, which are undoubtedly unprecedented and exclusive, further cast doubt on the validity of consent (a fundamental freedom) that is supposed to be free and informed, and which is said to have been given by the people who are now already vaccinated.
Every person has the right to clear, fair and appropriate information. This information is also perennial: if new data is revealed, those already vaccinated must be informed a posteriori (after the administration of this or that vaccine).
The “obligation” to vaccinate cannot therefore be sustained, even in a disguised form, notably through a “vaccine passport.”
This new analysis further confirms our previous reflections such as the one entitled “Could the Covid-19 vaccine (Tozinameran; COMIRNATY°) be qualified as ‘defective’ by a judge?” or those expressed in the two open letters that have already been sent to the Minister of Solidarity and Health and to the seven Orders of health professionals.
Vulnerability does not only arise from the age and state of health of individuals. Not being able to access independent information on medicines (including vaccines) is the first form of poverty and inequality.
Moreover, concerning the uncertainties on the effectiveness of these vaccines, the Council of State noted, on March 3, 2021, in particular the admission of the Ministry of Solidarity and Health itself, and the contradictions of the French “administration.” In this decision, and against the opinion of this Ministry, the Council of State had produced a decision that seemed to tend towards the recognition of this effectiveness. But, a few days later, in a new decision (n° 450413) issued on March 11, 2021, the Council of State changed its position and admitted “the uncertainty that remains regarding the real effectiveness of the vaccine in terms of the spread of the virus.” It should also be recalled that, on February 18, 2021, the Minister of Solidarity and Health also recognized, and that publicly, that no European country has been able to provide proof that these vaccines can prevent “severe” forms of COVID-19 (see press conference, starting at 34min 44s).
In its latest “Update on the surveillance of COVID-19 vaccines — Period from 12/03/2021 to 18/03/2021” published on March 26, 2021, and updated on March 29, 2021, the French National Agency for the Safety of Medicines (ANSM) reports, in particular, the number of deaths that have occurred in France after the administration of these vaccines. Deaths that are notified (reported) in pharmacovigilance (regardless of the certainty of the “causal link” between these vaccines and these deaths): “311 deaths” after administration of the BioNTech/Pfizer vaccine; “4 deaths” after administration of the Moderna vaccine; “20 deaths” after administration of the Astra Zeneca vaccine; (no data is available at this time regarding the latest vaccine (Janssen) to be licensed). In general, for all drugs, there is a high level of under-reporting in pharmacovigilance despite the mandatory nature of these reports.
Consequently, prudence would even dictate that, in all countries where these vaccines against COVID-19 have been marketed, all the batches thus “released” should be withdrawn immediately; and that these MAs that have been granted should be suspended, or even cancelled, as a matter of urgency until further notice. In any case, this is the sense of the recommendations that we could suggest to the ad hoc authorities, and in particular to the French authorities. And, at the very least, this information must be made known to everyone in a clear, fair, and appropriate manner.
All the more so since, in the case of serious adverse effects, including deaths, and in order to establish the said “causal link” with certainty, the victims and their families are often powerless when faced with the requirement of “probatio diabolica” [a legal requirement to achieve an impossible proof].
Astrazeneca, Coronavirus Vaccine, Ctiap, Johnson & Johnson, Moderna, Pfizer, Vacc
Keep this news available to you and millions more
Your gift will spread truth, defeat lies, and save lives
- Pastor Artur Pawlowski, arrested for refusing to shut church, tells allBlogs By John-Henry Westen
- Vatican turns synod on ‘synodality’ into yearlong international process with lay participationNews By Dorothy Cummings McLean
- Nigel Farage: Judeo-Christian West under threat from China, Marxism, cancel cultureNews By Stephen Kokx
- Frontline doctors file motion to stop FDA authorization of COVID vaccines for childrenNews By Patrick Delaney

Horrifying study reveals mRNA vaccine nanoparticles are circulated throughout the entire body: Brain, heart, liver, ovaries, testes and more – NaturalNews.com
Wednesday, June 02, 2021 by: Mike Adams
Tags: badhealth, badmedicine,
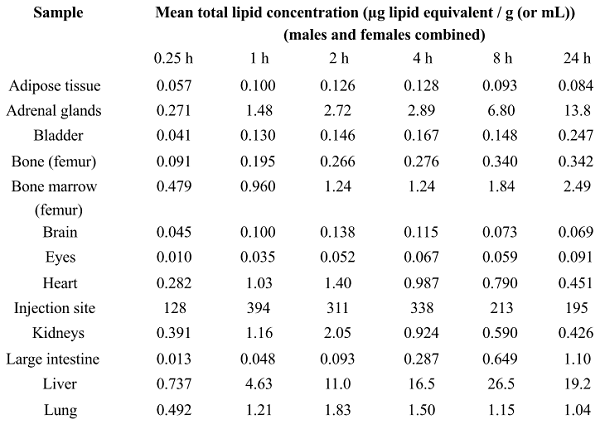
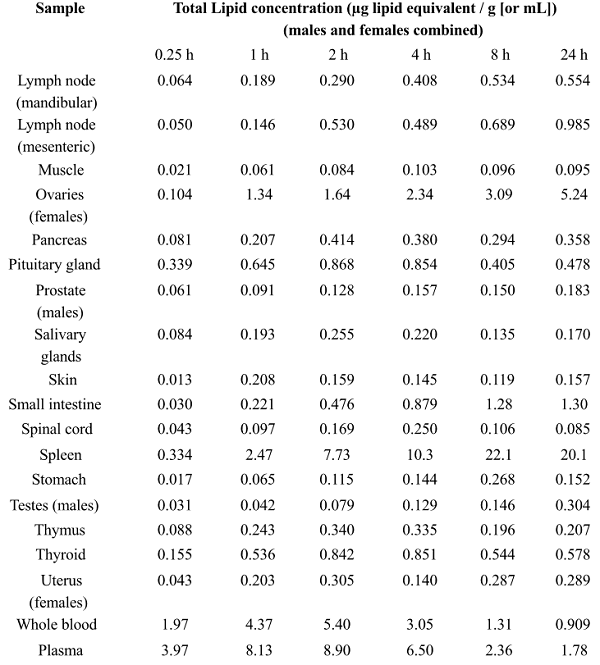
(Natural News) Not surprisingly, everything the establishment tells us about covid vaccines has been a calculated lie. One of the biggest and most treacherous lies is that “mRNA vaccine shots stay in the arm and don’t circulate nanoparticles around the body.” Now we know that is a complete lie, as new research conducted in Japan shows that Lipid NanoParticles (LNPs) containing the mRNA code are widely circulated around the body after vaccination, reaching the brain, spleen, large intestine, heart, liver, lungs and other organs.
The study paper, originally written in Japanese and auto-translated into English, can be found at this link on Natural News servers (PDF).
Labeled, “Pfizer confidential,” the study is known as a bio-distribution study that uses luciferase enzymes and radioisotope markers to accurately track the distribution of Pfizer’s mRNA LNPs across the body. The first section is labeled:
SARS-CoV-2 mRNA Vaccine (BNT162, PF-07302048)
2.6.4 Summary of pharmacokinetic study
The study reveals how mRNA LNPs are distributed across the body, even affecting ovaries and testes, raising huge questions about fertility in those receiving mRNA vaccine shots. The following chart shows the mass of NLPs (in micrograms) found in each organ following mRNA vaccination. Notice how it attacks the adrenals?
Pro-vaccine doctor raises the alarm bells: “We made a big mistake.”
A pro-vaccine doctor named Dr. Byram W. Bridle, PhD. was interviewed by Alex Pierson of the “On Point” podcast. That interview is also mirrored on Brighteon.com and shown here. It is approximately 9 minutes in duration:
Brighteon.com/3d683a15-fc3d-
In the interview. Dr. Bridle says the following stunning things. Remember as you read this that Dr. Bridle is 100% pro-vaccine and has no criticism about other vaccines: (emphasis ours)
…the spike protein, on its own, is almost entirely responsible for the damage to the cardiovascular system. If it gets into circulation, indeed, if you inject the purified spike protein into the blood of research animals, they get all kinds of damage to the cardiovascular system, and it can cross the blood-brain barrier, and cause damage to the brain.
They found the spike protein in circulation, so in the blood of 11 of those 13 healthcare workers that had received the vaccine. What this means is, so we have known for a long time that the spike protein is a pathogenic protein. It is a toxin. It can cause damage in our body if it gets into circulation. Now, we have clear cut evidence that the vaccines that make our bodies or the muscles or the cells in our deltoid muscles, manufacture this protein, not the vaccine itself, plus the protein gets into blood circulation. When in circulation, the spike protein can bind to the receptors that are on our platelets and the cells that line our blood vessels. When that happens, it can do one of two things. It can either cause platelets to clump and that can lead to clotting. That’s exactly why we’ve been seeing clotting disorders associated with these vaccines. It can also lead to bleeding. And of course, the heart’s involved, it’s a key part of the cardiovascular system. That’s why we’re seeing heart problems. The protein, it can also cross the blood brain barrier and cause neurological damage. That’s why also in the fatal cases of blood clots, many times it’s seen in the brain.
In short, the conclusion is we made a big mistake. We didn’t realize it until now. We saw the spike protein was a great target antigen. We never knew the spike protein, itself, was a toxin and was a pathogenic protein. So, by vaccinating people, we are inadvertently inoculating them with a toxin, and in some people this gets into circulation. And when that happens in some people, it can cause damage, especially with the cardiovascular system. I don’t have time, but many other legitimate questions about the long-term safety there for this vaccine. For example, with accumulating in the ovaries, one of my questions is, “will we be rendering young people infertile, some of them infertile?” So, I’ll stop there.
Dr. Bridle is now saying exactly what I have been saying, of course. The spike protein is the toxin, and it is what’s causing blood clots and deaths.
Situation Update podcast reveals even more details
I discuss all this starting at 50:36 in today’s Situation Update podcast, which also covers biosludge pollution, vaccine news and crazy headlines such as gun lotteries to push vaccines in West Virginia.
Brighteon.com/abfb98c0-717b-
Tomorrow’s Situation Update podcast will discuss the bombshell Fauci emails that broke today, revealing Fauci knew about gain-of-function research, helped fund it, and tried to cover it up.
Find that podcast tomorrow at:
CCP planning major attack on USA this year: Bioweapons, cyber war, kamikazee drones and infrastructure sabotage – NaturalNews.com
Friday, June 04, 2021 by: Mike Adams
Tags: airfields, biological warfare, CCP, communist China, cyber warfare, deep state, drone wars, energy infrastructure, invasion, Joe Biden, military, national defense, national security, Pentagon, power grid, President Trump, sabotage, treason, Worl
(Natural News) Over the last 48 hours, I’ve received a flood of intel from sources, both private and public, indicating that communist China is moving up the timetable of a long-planned attack on the United States of America. In reality, the biological warfare phase of the attack is already under way, having begun in 2019 with the deliberate release of the SARS-CoV-2 coronavirus, followed by direct interference in rigging the 2020 election and installing CCP puppet Joe Biden as a temporary occupant of the White House.
Now, due to the rapid emergence of evidence showing Fauci conspired with the CCP to fund gain-of-function development and build a biological weapon that attacks human ACE2 receptor sites, China is accelerating its own timetable to take down the USA before such investigations can conclusively place the blame for covid on China’s communist regime.
According to my sources, the planned attack on America will consist of:
- Cyber warfare attacks on critical infrastructure such as energy, transportation, finance and the power grid.
- Drone kamikazee (kinetic) attacks on critical infrastructure to conduct acts of sabotage.
- The advancing of ground troops into Southern U.S. states in an attempt to occupy and defend FOBs (Forward Operations Bases) in Texas, New Mexico, Arizona and California. (This will happen after the power grid sabotage plunges America into chaos.)
The stealth drone that was recently spotted over Tucson, AZ, monitoring the Davis-Monthan AFB, is part of this effort. There are hundreds more stealth drones operating in U.S. air space right now, controlled by communist China, according to my ex-military sources.
As reported by The Epoch Times, Kyle Bass has also warned of the 130,000-acre parcel of land in Southwest Texas that is now owned by the same Chinese billionaire who owns two-thirds of the land in China’s Xinjiang region, where slave labor concentration camps are in full operation. From TET:
The land for the wind farm is owned by a Chinese company called GH America Investments Group, which has since 2015 bought 130,000 acres of land—an area the size of Tulsa, Oklahoma—in Val Verde County. The man behind the firm is Sun Guangxin, a businessman from the northwestern Xinjiang region in China, who has strong ties to the communist regime.
Sun, a former military officer, is currently the richest person in Xinjiang—where the regime is committing genocide against ethnic Muslim minorities. He has a net worth of $1.9 billion, according to Forbes, and was also the vice chairman of the Xinjiang Provincial Youth Federation.
The goal of this, I’m told, is that China plans to construct and operate concentration camps in Texas and other U.S. states, then use them for mass incineration and disposal of all the Americans they plan to kill.
China is also actively and loudly threatening America with a rapid expansion of its nuclear weapons capabilities, according to a recent report published by The Sun (UK):
“We must be prepared for an intense showdown between China and the US,” Hu wrote in a chilling op-ed for the Chinese state-run newspaper. “In that scenario, a large number of Dongfeng-41, and JL-2 and JL-3 (both intercontinental-range submarine-launched ballistic missile) will form the pillar of our strategic will.
Seven months ago, I interviewed JR Nyquist who warns of this exact scenario, describing how China has prepositioned weapons, military uniforms and sabotage equipment in the United States, ready for activation:
In today’s Situation Update podcast, I provide additional frightening details about China’s attack plans and why they are now being accelerated to be activated this year. For example, one of my contacts informed me that an airfield being constructed by China in Texas is slated for completion by the end of July, with expected usage of the 10,000-foot runway by August or September.
A 10,000-foot runway is only needed for extremely large military cargo planes, yet this is a private airfield being constructed in Texas by communist China.
It begs the question: Why is the Pentagon under Joe Biden allowing foreign enemies to construct airfields in the United States? It seems insane. China is invading the United States without firing a single shot, and since China now controls the White House, the media, Hollywood and even Fauci, there seems to be no authority remaining in America that will work to defend the nation against a foreign invasion.
The timetable now appears to be just months away…
Brighteon.com/f8aacdc0-0980-
Find a new Situation Update podcast each day at:












 By Jeanne Smits, Paris correspondent
By Jeanne Smits, Paris correspondent